Mastering biocidal products regulation: a guide for regulatory affairs consulting
The Biocidal Products Regulation (BPR) (EU 528/2012) plays a crucial role in ensuring that biocidal products are safe for humans, animals and the environment. However, for businesses in the biocide industry, navigating this complex regulatory framework can be challenging. Regulatory affairs consulting professionals help companies stay compliant while ensuring a smooth and efficient product registration process.
In this guide, we’ll explore the key aspects of biocidal products regulation, the challenges businesses face and how regulatory affairs consulting services can streamline compliance, reduce risks and accelerate market access.
Understanding biocidal products regulation (BPR)
The BPR governs the approval, sale and use of biocidal products in the European Union and the UK. Its goal is to ensure that these products are effective while minimising risks to people, animals and the environment.
Key elements of the BPR
- Active substance approval – all active substances used in biocidal products must be approved before the product itself can be authorised
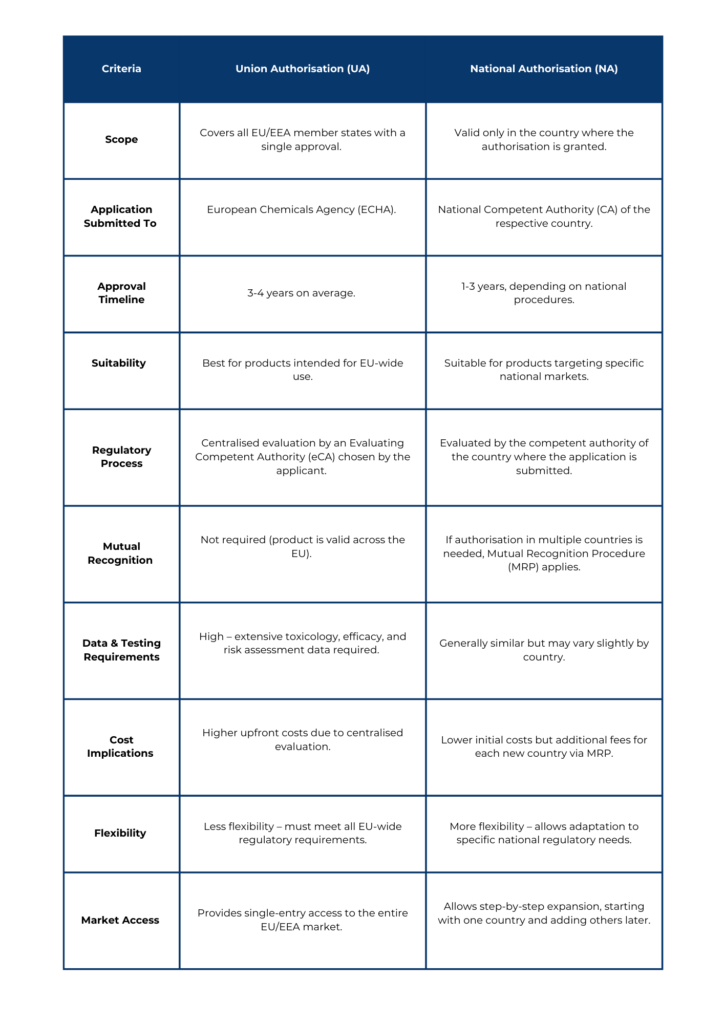
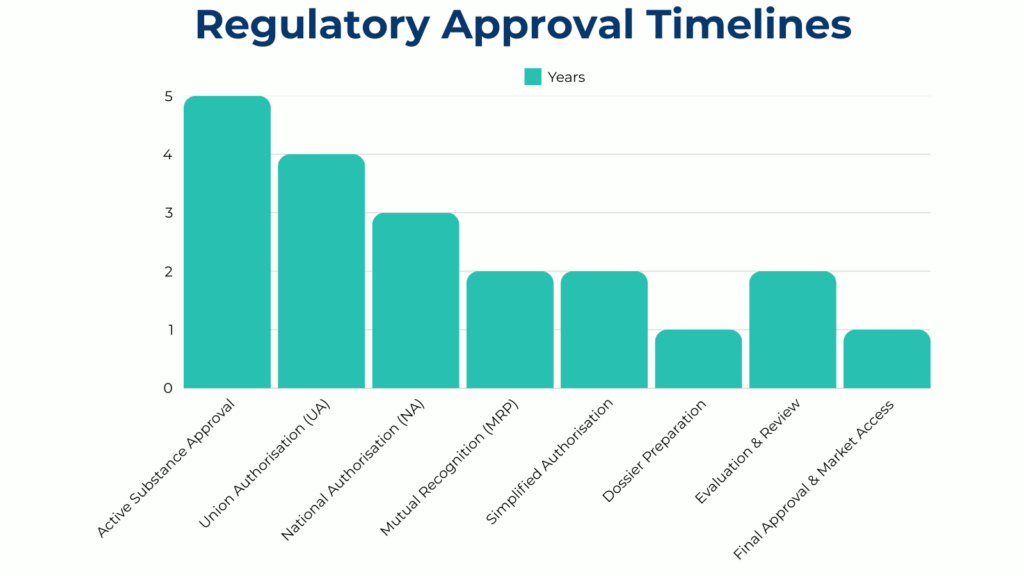
- Product authorisation – companies must obtain approval for each biocidal product before placing it on the market. This can be done at the national or EU-wide level
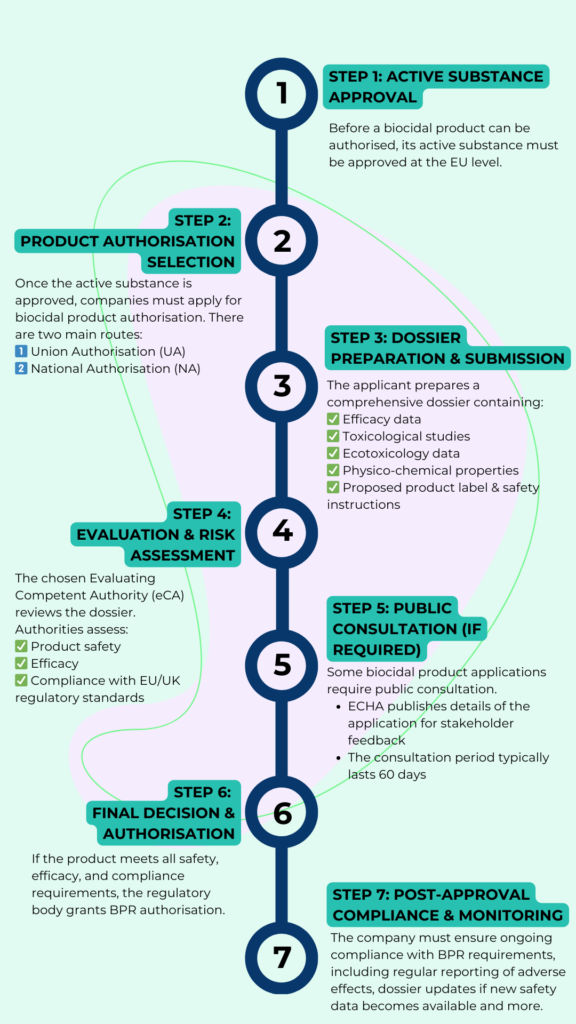
- Union authorisation vs. national authorisation – companies can apply for EU-wide approval through union authorisation or choose national authorisation for individual member states
- Ongoing compliance – once a product is approved, manufacturers must ensure continued compliance, including dossier renewals and adherence to updated safety standards
Recent regulatory updates and their impact
📌 Changes in active substance approvals – new criteria for evaluating environmental and health risks
📌 UK BPR divergence – since Brexit, UK biocide regulations now require separate applications for Great Britain (GB) and the EU
📌 Data sharing and ECHA decisions – the European Chemicals Agency (ECHA) enforces stringent requirements for data submissions, leading to potential delays in approvals
How regulatory affairs consulting supports compliance
Navigating biocidal products regulation requires in depth knowledge of compliance requirements, dossier preparation and regulatory submissions. This is where regulatory affairs consulting firms provide invaluable support.
Key benefits of working with regulatory affairs consultants
✅ Expert guidance on dossier preparation – ensuring that all required data (toxicology, efficacy, risk assessments) is properly compiled and formatted
✅ Regulatory strategy development – identifying the most efficient approval pathway (e.g. Union vs. National Authorisation)
✅ Method development and validation – creating and verifying analytical methods to ensure compliance with regulatory standards
✅ Liaison with regulatory authorities – handling communications and negotiations with ECHA, national competent authorities and the UK Health & Safety Executive (HSE)
✅ Post approval compliance management – supporting companies in maintaining compliance after product authorisation, including regulatory monitoring and updates
🚀 By partnering with a regulatory affairs consultant, businesses can reduce approval time, minimise compliance risks and confidently expand into new markets.
Challenges in BPR compliance and how to overcome them
Despite the structured regulatory framework, many companies face significant hurdles in achieving compliance.
Common challenges:
❌ Regulatory complexity – interpreting ever-changing rules and requirements can be overwhelming
❌ Data requirements – companies must provide extensive toxicology, ecotoxicology and efficacy data
❌ Approval delays – lengthy regulatory review processes can delay market entry
❌ High compliance costs – generating required safety and efficacy data can be expensive
❌ Diverging UK & EU regulations – separate applications are now required post-Brexit
Solutions for success:
✔️ Start early – engage regulatory consultants at the beginning of the product development process
✔️Develop a clear strategy – choose the right approval pathway based on market needs
✔️ Ensure robust testing & data – work with accredited laboratories for high quality data submissions
✔️ Method validation for compliance – ensure testing methods meet regulatory standards to avoid submission rejections
✔️ Monitor regulatory updates – stay informed about policy changes and industry trends
✔️ Leverage expert support – partner with regulatory affairs consulting firms for guidance and risk mitigation
💡 Did you know?
Delays in regulatory approvals can cost companies millions in lost revenue. By streamlining compliance, businesses can gain a competitive advantage.
Emerging trends in biocidal product regulation
The regulatory landscape for biocidal products is continuously evolving. Manufacturers and regulatory affairs consulting professionals must stay ahead of these changes to maintain compliance and competitive advantage. Here are some key trends shaping the industry:
1️⃣ Stricter environmental & safety regulations
Regulatory agencies are implementing more stringent risk assessments to ensure that biocidal products pose minimal risks to human health and the environment. This includes:
- Tighter controls on active substances
- Enhanced toxicity and ecotoxicity testing requirements
- Increased restrictions on volatile organic compounds (VOCs) in formulations
✅ What this means for businesses:
Companies must focus on method development and method validation to meet the latest safety standards efficiently.
2️⃣ Demand for sustainable biocidal products
With growing pressure to reduce environmental impact, there is an increasing demand for:
- Bio-based alternatives to synthetic chemicals
- Biodegradable and eco-friendly formulations
- Reduced-residue plant protection solutions
✅ What this means for businesses:
Companies investing in sustainable product formulation will have a competitive edge in regulatory approvals and market preference.
3️⃣ Regulatory divergence between the UK and EU
Since Brexit, the UK has introduced its own BPR framework, leading to:
- Separate dossier submissions for EU BPR and UK BPR
- Different timelines for active substance renewals
- Variations in testing and validation requirements
✅ What this means for businesses:
Regulatory teams must tailor compliance strategies separately for UK and EU markets to avoid approval delays.
4️⃣ Advances in digital compliance tools
Regulatory bodies and businesses are increasingly adopting digital platforms to streamline compliance:
- AI-driven risk assessments to predict regulatory challenges
- Blockchain for traceability of regulatory approvals
- Automated dossier submissions to reduce human errors
✅ What this means for businesses:
Companies should integrate regulatory technology (RegTech) solutions to enhance efficiency and accuracy in compliance processes.
Best practices for a smooth biocide product approval process
🔹 Engage with regulators early to clarify submission requirements
🔹 Ensure high quality data to support efficacy and safety claims
🔹 Invest in method validation to ensure accurate and reliable test results
🔹 Work with a regulatory affairs consulting partner for expertise and efficiency
🔹 Stay up to date with evolving regulatory frameworks
📢 Need expert guidance? Contact Oxford Analytical Services today to ensure seamless compliance with biocidal products regulation.
Final thoughts
Successfully navigating biocidal products regulation requires expertise, strategic planning, and access to high quality regulatory affairs consulting. By following best practices and leveraging professional support, companies can streamline compliance, reduce costs and accelerate time to market.
🚀 Get in touch with our regulatory experts today and take the stress out of BPR compliance!

To download this guide as a helpful pdf, complete the short form below.

